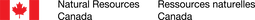SUPRAMOLECULAR CYANOMETALLATE COORDINATION POLYMER MATERIALS
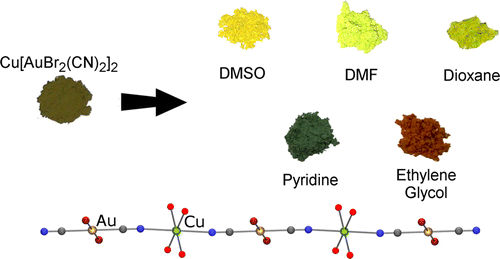
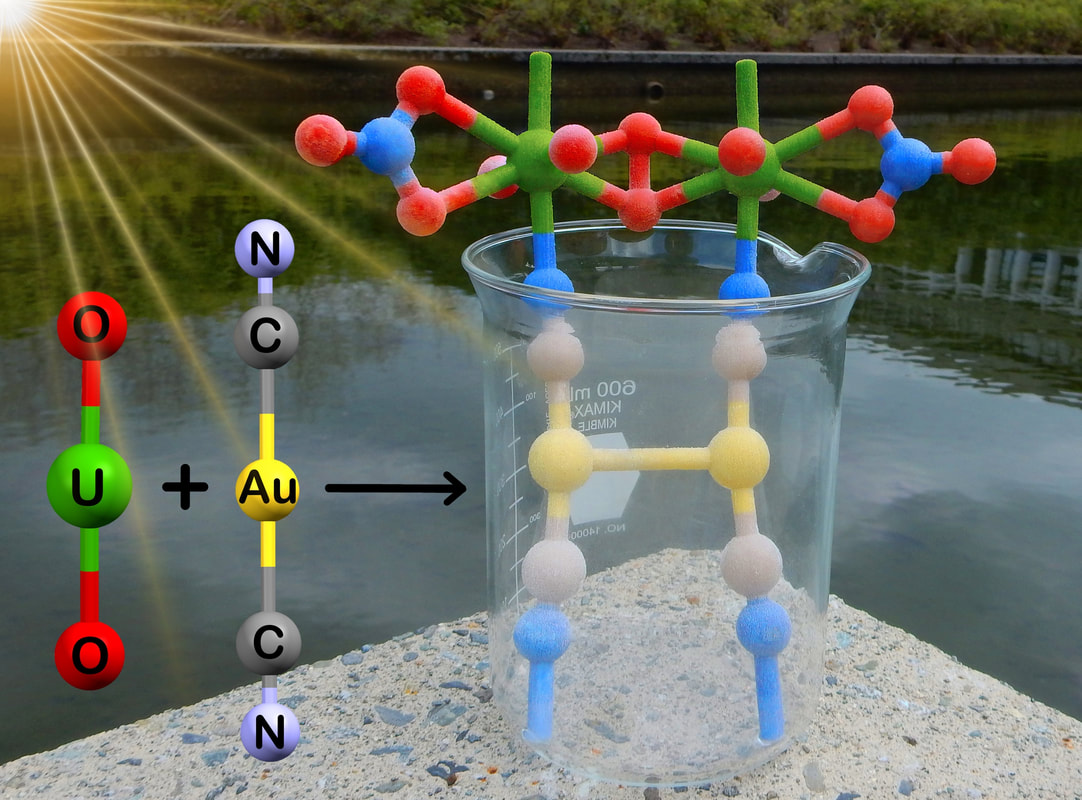
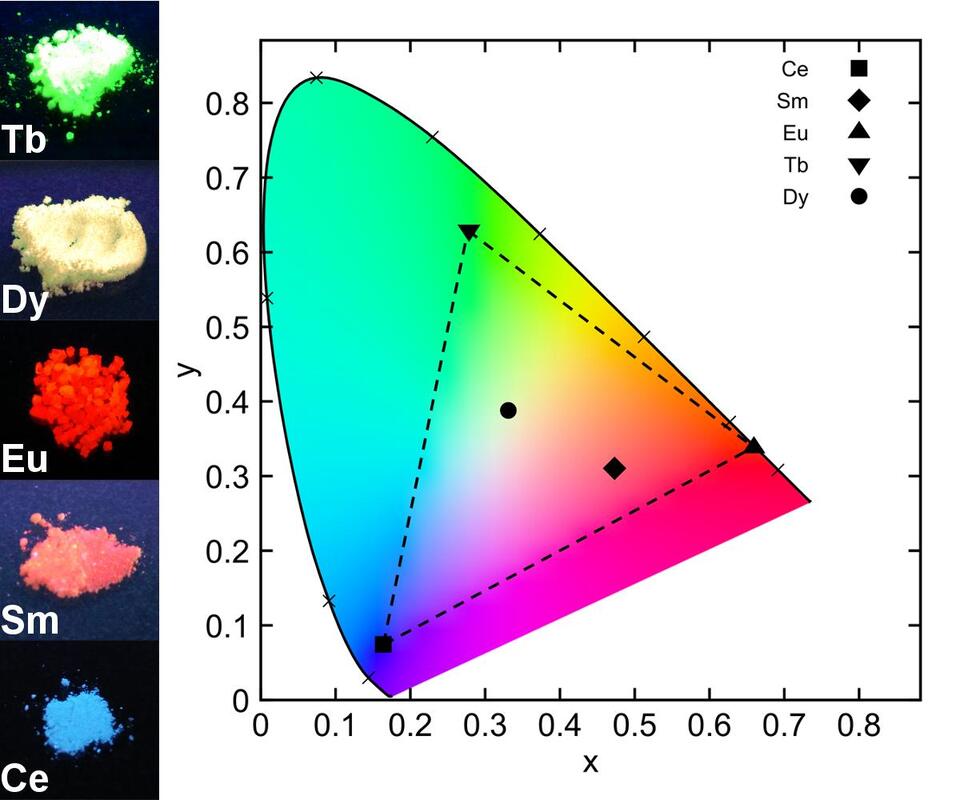
Using combinations of transition metals, lanthanides, actinides and appropriate bridging ligands, we are pursuing novel coordination polymer materials. Bridging ligands include metal cyanides and thiocyanates, heterocyclic amines and sulfur-based ligands. In particular, we often use linear d10 and square-planar d8 cyanometallates such as [Au(CN)2]- and [Pt(CN)4]2- that display strongly attractive metal-metal (aurophilic, platinophilic etc.) interactions in order to make new materials. We are targeting emissive and vapochromic materials for toxic small-molecule gas sensing (e.g. ammonia), highly birefringent or dichroic materials, molecule-based magnetic and multifunctional materials, systems with unusual thermal-expansion properties, supramolecular systems that emit white or other-coloured light, and coordination polymer materials with other exciting properties. A separate section focusing on our f-element coordination polymers is below.
Representative Publications:
Representative Publications:
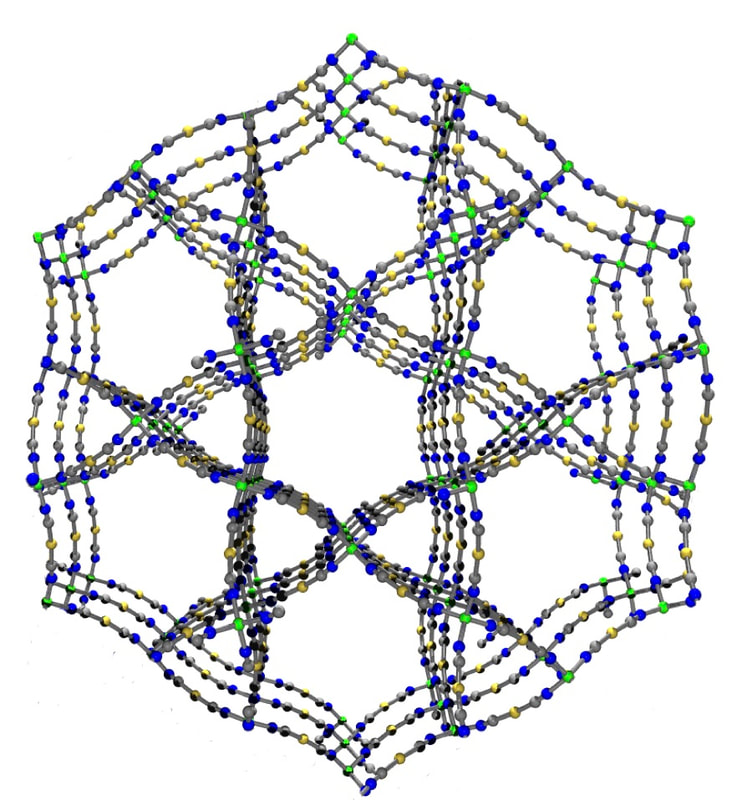
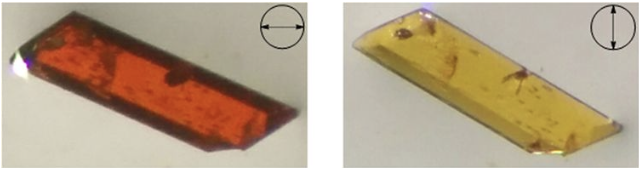
- Karpiuk, T.E.; Leznoff, D.B. "Anisotropic thermal expansion of structurally related lanthanide-mercury(II) cyanide coordination polymers, Inorg. Chem., 2024, 10.1021/acs.inorgchem.3c03002
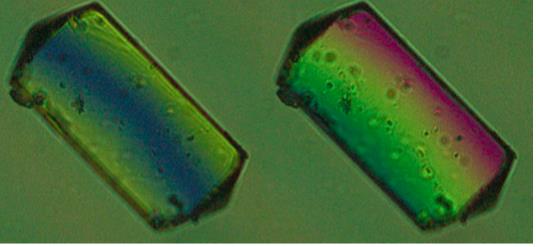
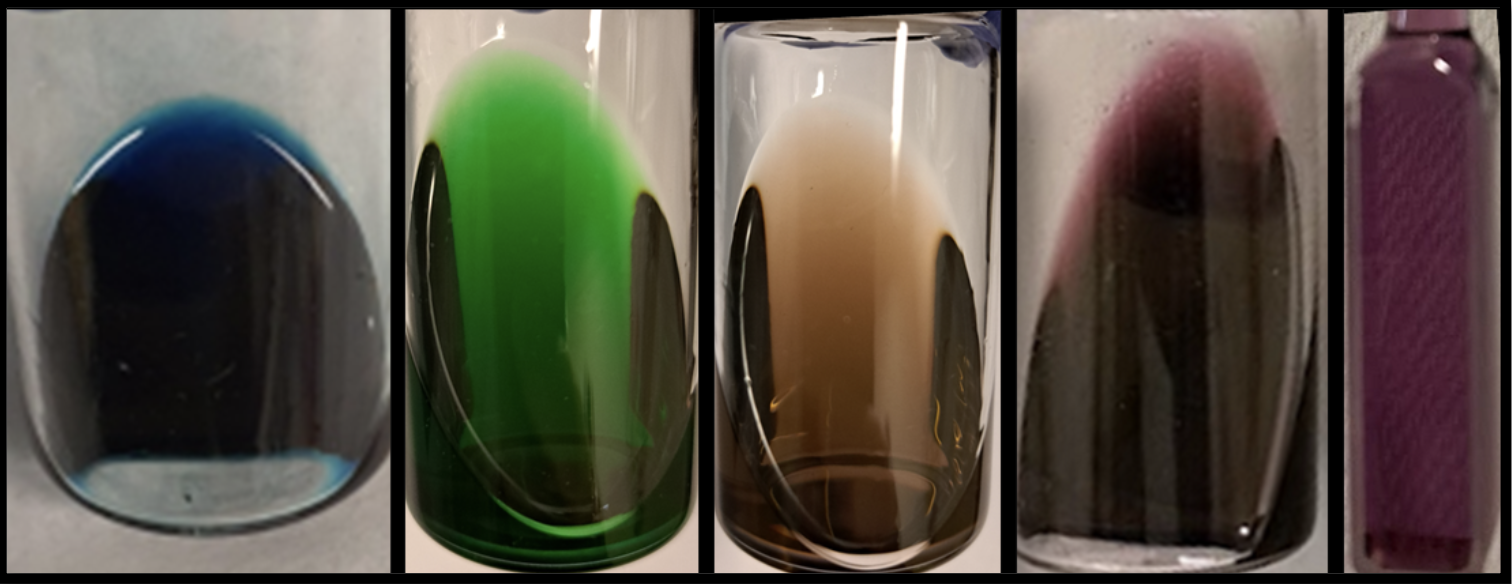
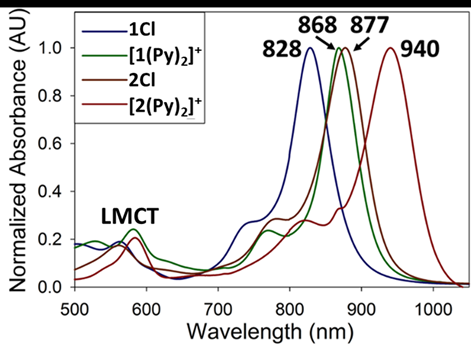
- Sergeenko, A.S.; Paripovic, D.; Bélanger-Desmarais, N.; Reber, C.; Leznoff, D.B. “Dichroic palladium(II)-dithiolate coordination polymers”, Cryst. Growth Des., 2023, 23, 5812-5820.
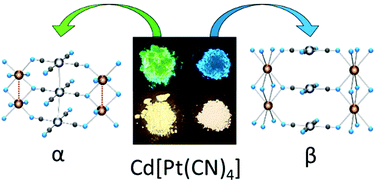
- Sergeenko, A.S.; Paripovic, D.; Dab, C.; Blanc, P.F.; Reber, C.R.; Leznoff, D.B. “Highly emissive polymorphs of anhydrous cadmium tetracyanoplatinate and their solvated coordination networks”, Dalton Trans., 2022, 51, 9531-9540.
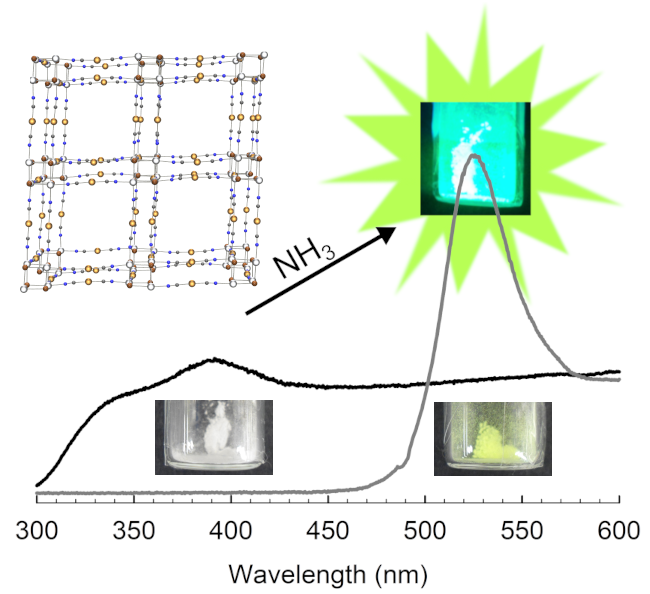
- Varju, Bryton R.; Wollschlaeger, S.; Leznoff, D.B. “Zinc(II) Tetracyanoplatinate: A reversible luminescence-based ammonia sensor”, Chem. Eur. J., 2019, 25, 9017-9025.
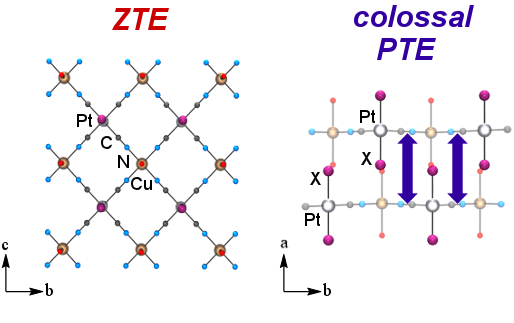
- Sergeenko, A.S.; Ovens, J.S.; Leznoff, D.B. “Designing Anisotropic cyanometallate coordination polymers with zero thermal expansion in two dimensions”, Chem. Commun., 2018, 54, 1599-1602.
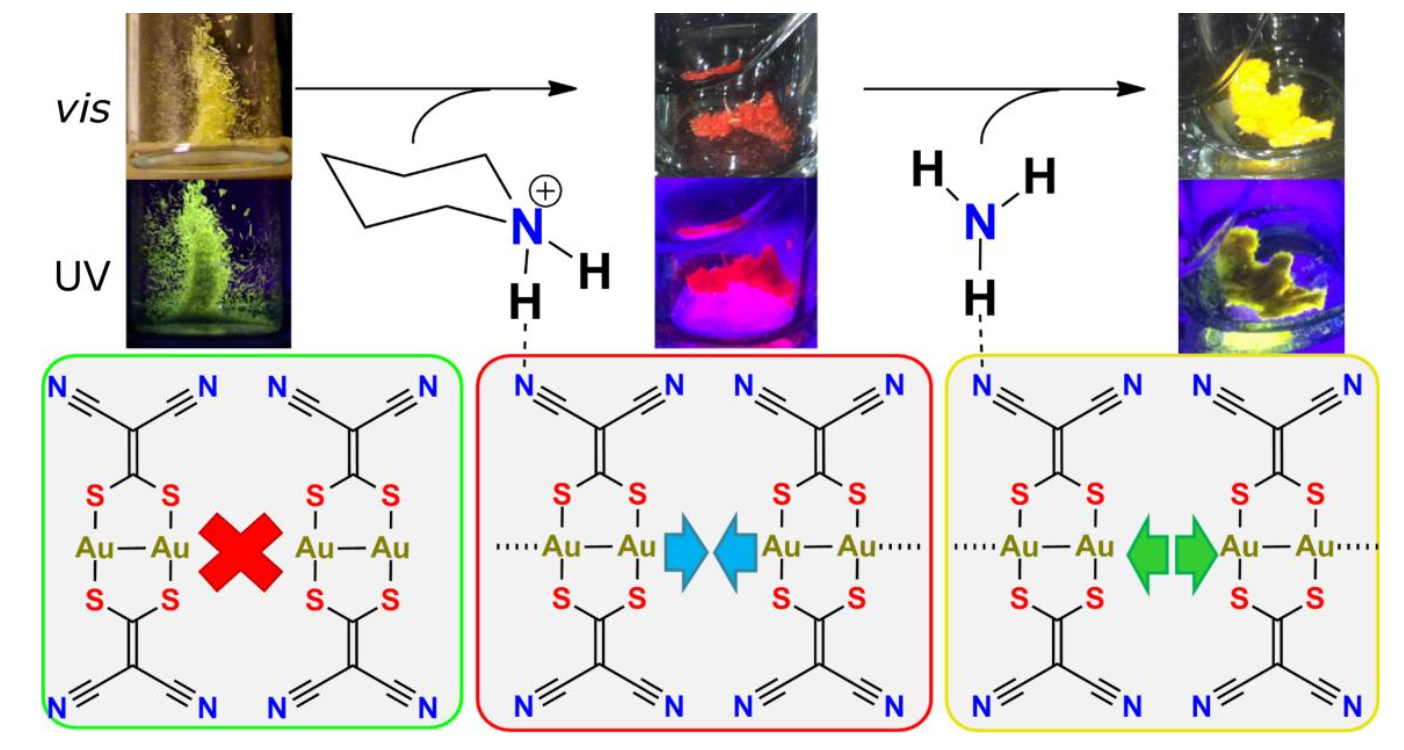
- Varju, B.R.; Ovens, J.S.; Leznoff, D.B. “Mixed Cu(I)/Au(I) coordination polymers as reversible turn-on vapoluminescent sensors for volatile thioethers”, Chem. Commun., 2017, 53, 6500-6503.
- Thompson, J.R.; Katz, M.J.; Williams, V.E.; Leznoff, D.B. "Structural design parameters for highly birefringent coordination polymers", Inorg. Chem., 2015, 54 6462-71.
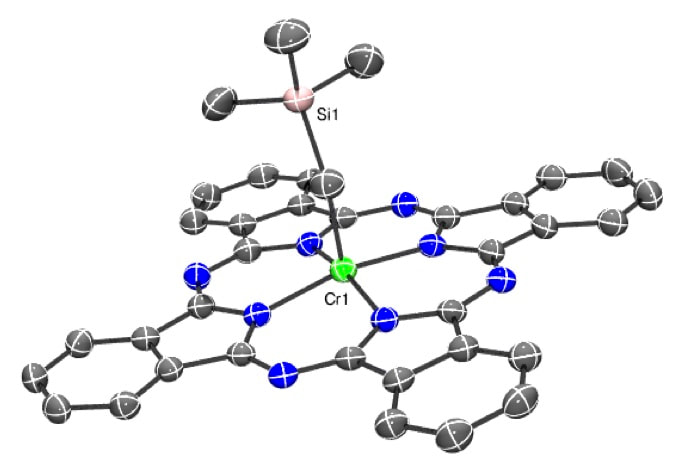
NON-TRADITIONAL METALLOPHTHALOCYANINESPhthalocyanines are extremely important industrial dyes that are highly chemically stable and have among the highest extinction coefficients known; they are also redox-active. They have a wide range of applications, including their use as blue/green dyes (such as "Phthalo-blue, the background colour of this website!), optical components, read-write optical memory, solar cells, catalysis and photodynamic therapy of cancer. However, a large majority of PcM complexes incorporate late first-row transition-metals. We focus on rare PcM complexes with early-transition and f-block metals. We probe their ability to act as redox-active ligands by isolating their ring-reduced/oxidized species, and use X-ray structures and spectroscopy to understand their properties. The overall goals are to harness these non-traditional PcM complexes for small-molecule activation (especially catalytic electroreduction of CO2 and other key molecules), as catalysts (e.g. for lactide polymerization), as magnetic materials, and as other advanced optical materials such as visible and Near-IR dyes that take advantage of their huge extinction coefficients. The exploration of organometallic compounds based on PcM complexes is also of particular interest, given the minimal overlap between these two huge fields of chemistry.
Representative Publications:
|
ACTINIDE and LANTHANIDE COORDINATION POLYMERS AND COMPLEXES
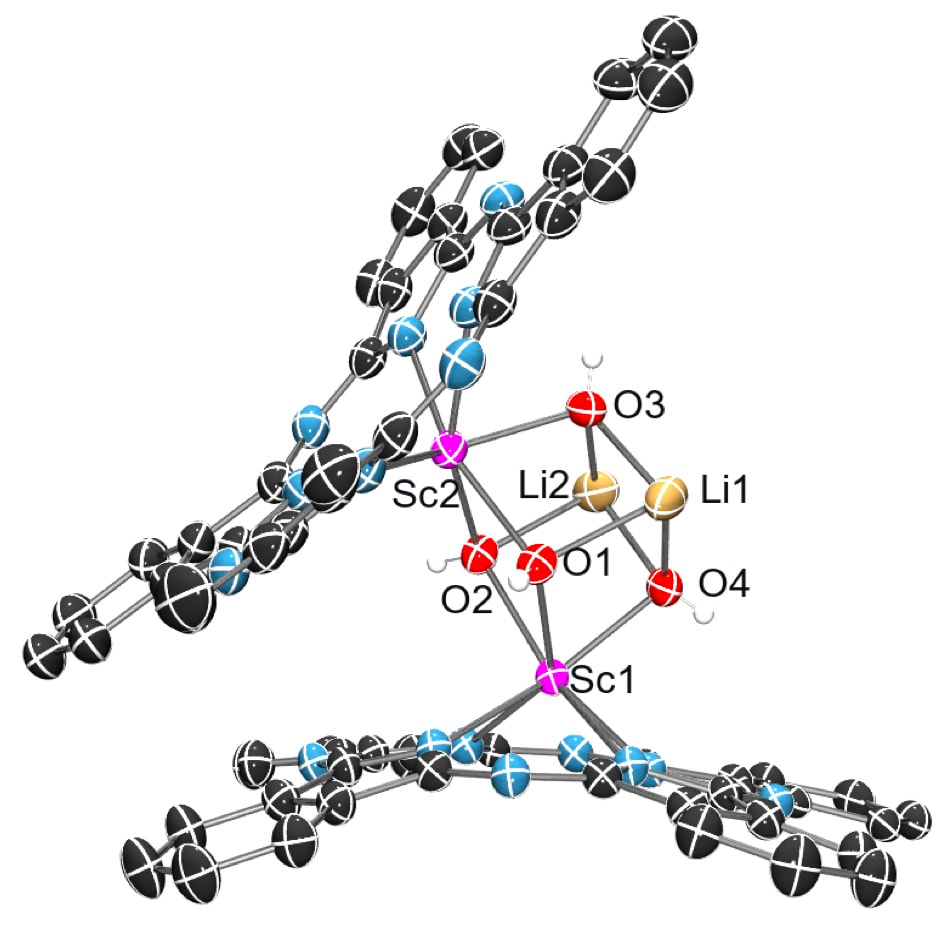
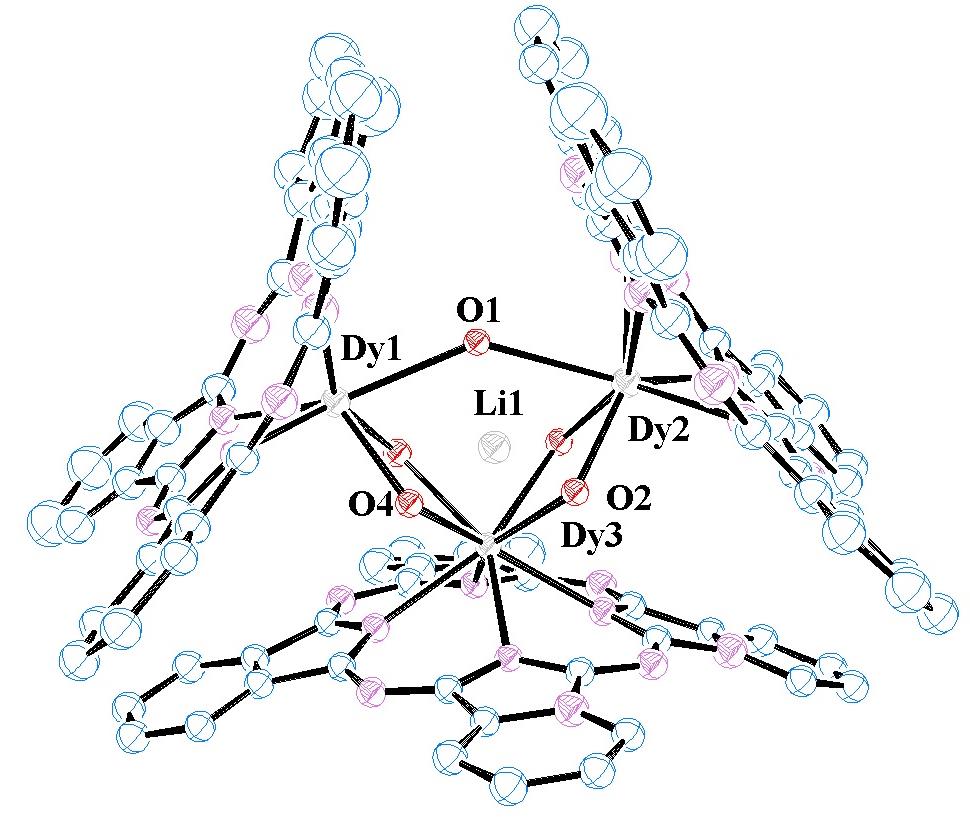
As an extension of our research in both supramolecular coordination chemistry and phthalocyanine work, the chemistry of thorium(IV) and uranium complexes from oxidation state +3 to +6, with a variety of ligand systems is explored. Actinide complexes with phthalocyanines, other chelating amido ligands and incorporation into cyanometallate coordination polymers are all being pursued. Actinides are relatively under-examined compared with their transition-metal counterparts and provide an exciting route into some unusual chemistry. We are also very active in the non-radioactive f-block...i.e., using lanthanides in coordination polymers and phthalocyanine materials, to harness their unusual structural and reactivity chemistry as well as their emissive and magnetic properties.
Representative Publications:
|
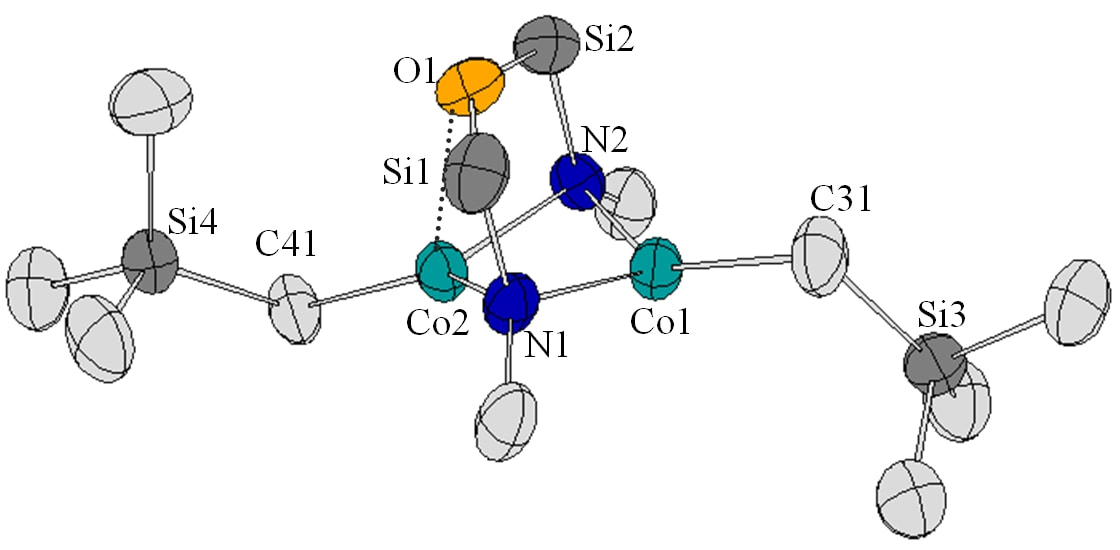
PARAMAGNETIC ORGANOMETALLICSParamagnetic organometallic complexes have been much less studied relative to the vast literature on diamagnetic organometallic complexes. We are interested in the synthesis, structural chemistry, reactivity and magnetic behaviour of this underexamined class of organometallics. To what extent does the presence of unpaired electrons at the metal centre affect the stability, structure and reactivity of these compounds? Reactivity studies in particular with respect to catalysis and redox chemistry are explored. Our phthalocyanines, as well as chelating diamido ligand platforms are utilized in this work.
Representative Publications:
|
Proudly powered by Weebly